Neutralisation
Aim: To observe a neutralisation reaction.
Equipment: A test tube, test tube rack, 1mol per liter sodium carbonate, 1mol per liter hydrochloric acid, dropper or dropper bottle, Universal indicator solution.
method:
1. Add approximately 1-2 mL of sodium carbonate and place the test tube into the test tube rack. Add 3-5 drops of Universal indicator solution
2. Using a dropper bottle, add hydrochloric acid drop by drop. be careful because adding even a small amount of extra acid can mean you'll miss the neutralisation point.
Observations
The only change that does produce something different during the neutralisation is the reaction between hydrogen ions and hydroxide ions, which produces water molecules. This is the ionic equation that represents the neutralisation reaction between any acid and any alkali.
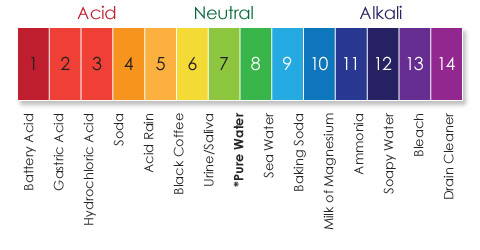
The pH of 1-3 is red and it's strong Acid, from 4-5 is orange and it's weak and 6 is yellow and its very very slightly acidic and 7-8 is green and it's neutral and 9-11 is blue and it's slightly basic and from 12-14 is purple and it's strongly acidic.
The only change that does produce something different during the neutralisation is the reaction between hydrogen ions and hydroxide ions, which produces water molecules. This is the ionic equation that represents the neutralisation reaction between any acid and any alkali.
The pH of 1-3 is red and it's strong Acid, from 4-5 is orange and it's weak and 6 is yellow and its very very slightly acidic and 7-8 is green and it's neutral and 9-11 is blue and it's slightly basic and from 12-14 is purple and it's strongly acidic.
No comments:
Post a Comment
To support my learning I ask you to comment as follows:
1. Something positive - something you like about what I have shared.
2. Thoughtful - A sentence to let us know you actually read/watched or listened to what I had to say
3. Something thoughtful - how have you connected with my learning? Give me some ideas for next time or ask me a question.
Note: only a member of this blog may post a comment.